Heat treatment is the process of heating and cooling metals, using specific predetermined methods to obtain desired properties. Both ferrous as well as non-ferrous metals undergo heat treatment before putting them to
use. Over time, a lot of different methods have been developed. Even today, metallurgists are constantly working to improve the outcomes and cost-efficiency of these processes.
For that they develop new schedules or cycles to produce a variety of grades. Each schedule refers to a different rate of heating, holding and cooling the metal.
These methods, when followed meticulously, can produce metals of different standards with remarkably specific physical and chemical properties.

The Benefits:
There are various reasons for carrying out heat treating. Some procedures make the metal soft, while others increase hardness. They may also affect the electrical and heat conductivity of these materials. Some heat treatment methods relieve stresses induced in earlier cold working processes. Others develop desirable chemical properties to metals. Choosing the perfect method really comes down to the type of metal and the required properties.
In some cases, a metal part may go through several heat treatment procedures. For instance, some super alloys used in the aircraft manufacturing industry may undergo up to six different heat treating steps to optimise it for the application.
Heat Treatment Process Steps:
In simple terms, heat treatment is the process of heating the metal, holding it at that temperature, and then cooling it back. During the process, the metal part will undergo changes in its mechanical properties. This is
because the high temperature alters the microstructure of the metal. And microstructure plays an important role in the mechanical properties of a material. The final outcome depends on many different factors.
These include the time of heating, time of keeping the metal part at a certain temperature, rate of cooling, surrounding conditions, etc. The parameters depend on the heat treatment method, type of metal and part size. Over the course of this process, the metal’s properties will change. Among those properties are electrical resistance, magnetism, hardness, toughness, ductility, brittleness and corrosion resistance.
Heating:

As we already discussed, the microstructure of alloys will change during heat treatment. Heating is carried out in line with a prescribed thermal profile. An alloy may exist in one of three different states when heated. It may either be a mechanical mixture, a solid solution, or a combination of both.
A mechanical mixture is analogous to a concrete mixture where cement binds sand and gravel together. Sand and gravel are still visible as separate particles.
With metal alloys, the mechanical mixture is held together by the base metal. On the other hand, in a solid solution, all the components are mixed homogenously.
This means that they cannot be identified individually even under a microscope. Every state brings along different qualities. It is possible to change the state through heating according to the phase diagram. The cooling, though, determines the final outcome. It is possible for the alloy to end up in one of the three states, depending solely on the method.
Holding:
During the holding, or soaking stage, the metal is kept at the achieved temperature. The duration of that depends on the requirements. For example, case hardening only requires structural changes to the surface of the metal in order to increase surface hardness. At the same time, other methods need uniform properties. In this case, the holding period is longer. The soaking time also depends on the material type and part size. Larger parts need more time when uniform properties are the objective. It just takes longer for the core of a large part to reach the required
temperature.
Cooling:
After the soaking stage is complete, the metal must be cooled in a prescribed manner. At this stage, too, structural changes occur. A solid solution on cooling may stay the same, become a mechanical mixture completely or partially, depending on various factors. Different media such as brine, water, oil or forced air control the rate of cooling. The sequence of cooling media named above is in decreasing order of effective rate of cooling. Brine absorbs heat fastest, while air is the slowest. It is also possible to use furnaces in the cooling process. The controlled environment allows for high precision when slow cooling is necessary.
Phase Diagrams:
Each metal alloy has its own phase diagram. As previously said, heat treatment is done according to these diagrams.
They show the structural changes that take place at different temperatures and different chemical compositions. Let’s use the iron-carbon phase diagram as an example, as this is the most known and widely taught one at universities.
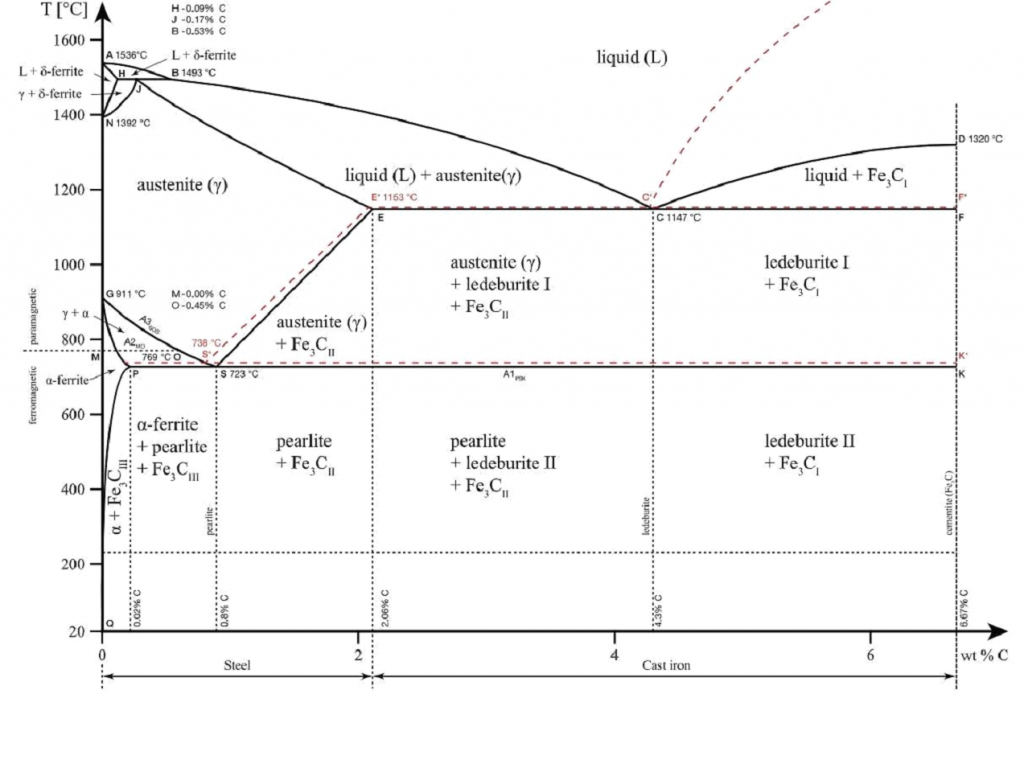
The iron-carbon phase diagram is an important tool when learning about the behaviour of different carbon steels when subjected to heat treatment. The x-axis shows the carbon content in the alloy and the y-axis shows the temperature. Note that 2.14% of carbon is the limit where steel becomes cast iron, The diagram displays various regions where the metal exists in different microstates such as austenite, cementite, pearlite. These regions are marked by boundaries A1, A2, A3, and Acm. At these interfaces, phase changes occur when the temperature or carbon content value passes through them.
A1: The upper limit of the cementite/ferrite phase.
A2: The limit where iron loses its magnetism. The temperature at which a metal loses its magnetism is also called Curie temperature.
A3: The interface that separates Austenite + Ferrite phase from the γ (Gamma) austenite phase.
Acm: The interface that separates γ Austenite from the Austenite + Cementite field.
The phase diagram is an important tool to consider whether heat treatment will be beneficial or not. Each structure brings along certain qualities to the final product and the choice of heat treatment is made based on that.



 فارسی
فارسی